Cell Division
But President Bush doesn’t like his solution. Which is why a growing number of stem cell researchers are turning to a new technique that circumvents the embryonic cloning debate altogether. Now, Mitalipov must decide whether to give up his secrets to another country or play political roulette.
Nestled within the razor-wire-capped, chain-link perimeter of the Oregon National Primate Research Center (ONPRC)—a suburban Jurassic Park that sits on 300 acres of forest in Beaverton—Oregon Health & Science University’s Embryonic Stem Cell Culture Laboratory is not much bigger than a walk-in closet. Inside, it’s uncomfortably warm, thanks to two thrumming incubators heated to 98.6 degrees Fahrenheit—the precise temperature of a mammalian uterus. Beneath overhead fluorescents, the lab’s head researcher, Shoukhrat Mitalipov, hunches over a microscope.
A short, effusively polite 46-year-old émigré from Kazakhstan with a boyish face and spiky black hair, Mitalipov is an emotive speaker, furrowing his eyebrows for emphasis and transposing his v’s as w’s just like Ensign Chekov in the original Star Trek series. Like a proud father, he’s eager to demonstrate what the embryonic stem cells growing inside his incubators can do.
“Now watch closely,” he says as he places a petri dish under the lens of his microscope, which is wired to a small flat-panel display. On the screen, the cellular clump looks vaguely disgusting, like a wad of phlegm on a sidewalk. But then the microscopic bit of spittle expands and contracts. Again, almost imperceptibly, it bulges out, then in.
Actually, Mitalipov informs me, it’s beating. As it should, because this is freshly minted heart tissue.
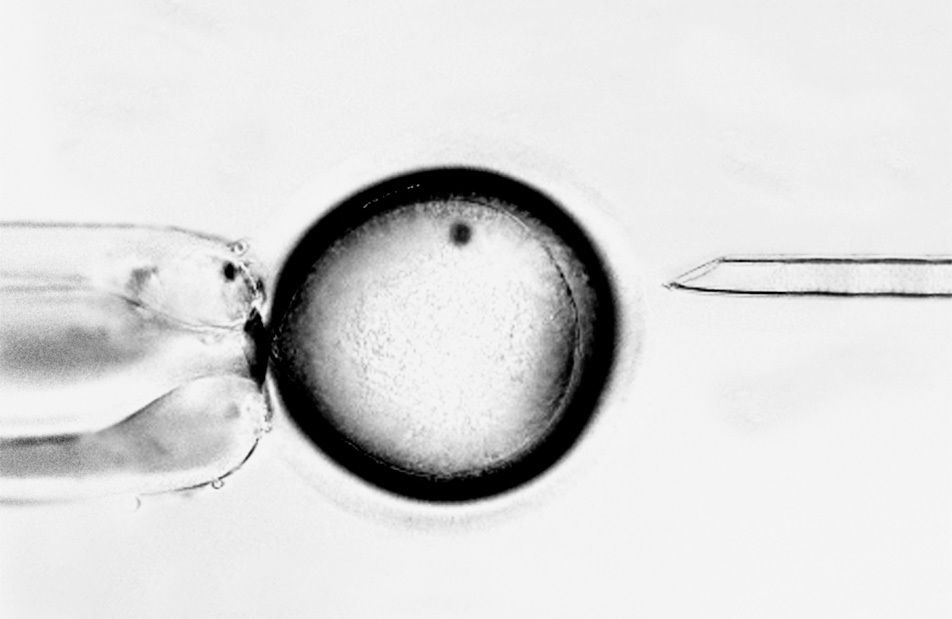
What’s incredible is that this freakish, living, pulsating lump of nascent heart started out as a skin cell from a monkey’s ear. By fusing the nucleus of that skin cell with an unfertilized monkey egg from which the DNA had been removed, Mitalipov created a new monkey embryo that’s genetically identical to the skin cell donor. A cloned monkey embryo, mind you—the first of its kind. A cloned monkey embryo that then yielded a colony of embryonic stem cells—universal cells that can morph into any cell type—during the embryo’s first weeks of development. And those stem cells grew into the heart tissue that’s currently pumping with life under the microscope.
It’s those cloned monkey embryos that, last November, made it to the cover of the journal Nature and transformed Mitalipov into a scientific hero. For, with the capability to clone monkey embryos, scientists were mere steps away from being able to clone human embryos. Meaning it might be possible to produce human stem cells that could sprout human heart tissue, which could theoretically replace the damaged heart tissue of a heart attack victim. Ditto with bone marrow for leukemia patients. Or neurons for those with Alzheimer’s.
But if that day comes, Mitalipov most likely will be publishing his results from another continent. Because one week after his feat was announced, the scientific hero became a kind of exile.
No one has accused him of any wrongdoing, per se. It’s just that in the Darwinian universe of stem cells funding, Mitalipov’s method of generating stem cells has been superseded by a competing method: One that doesn’t require the discarding or destruction of an embryo—a potential life, as some see it—whether human or primate. One that even Catholics and President Bush can get behind.
And so Mitalipov finds himself in a somewhat unsettling position. While his competitors are lavished with political support, multimillion-dollar grants and new facilities, he has received nothing—a situation that will force him either to adopt a more politically friendly line of research at OHSU, as many of his colleagues elsewhere already have, or take his procedure on the road, to another country.

Mitalipov’s scientific competitor is a reclusive researcher named Jamie Thomson, and it so happens that the two share a bit of history: From 1989 to 1991, seven years before Mitalipov arrived, Thomson worked as a postdoc at the Oregon National Primate Research Center. Recognizing Thomson’s tremendous talents as a scientist, Don Wolf, the head of the primate center’s embryology research group at the time, attempted to convince OHSU to hire the postdoc permanently in 1991, but administrators, says Wolf, weren’t interested in keeping Thomson on.
“He was accomplished beyond his years,” says Wolf, still frustrated. “There was absolutely no question that he was going to be hugely successful in the future.”
Thomson became a scientific superstar in 1998, a heady time for the fledgling field that had been dubbed “bioengineering.” Two years earlier, an Edinburgh embryologist named Ian Wilmut had figured out how to clone an animal. Using a process called “somatic cell nuclear transfer”—the same process that Mitalipov would later refine in monkeys—Wilmut took an egg from a Scottish blackface ewe and swapped out its DNA with the DNA from a 6-year-old sheep of a different breed, a Finn Dorset. After zapping the egg with a jolt of electricity to initiate cell division, Wilmut implanted the newly formed embryo into another blackface ewe’s uterus. Five months later, a fuzzy, perfectly healthy little lamb was born, a baby clone of the adult Finn Dorset. Wilmut named the cloned lamb “Dolly.”
Dolly’s birth announcement on February 23, 1997, tapped into the public’s worst fears about cloning. It didn’t help that Wilmut’s work was funded by a biotech firm interested in bioengineering sheep to produce milk laden with a protein commonly used in pharmaceuticals. In interviews with the press, Wilmut attempted to dispel worries that Dolly was a prelude to human cloning.
“Why would you want to make another human being?” Wilmut asked Malcolm Ritter, an Associated Press science writer. “It would be ethically unacceptable.”
Nevertheless, the headlines that followed exuded an air of Aldous Huxley’s Brave New World, presaging legions of identical alphas and betas. In its lead story, the New York Times quoted Lee Silver, a Princeton biology professor, who declared, “It means all science fiction is true.”
In response, President Bill Clinton convened the National Bioethics Advisory Commission, which recommended imposing a five-year moratorium on government funding of human cloning research, a ban that George Bush extended once in office. It stands to this day.
As portentous a development as Dolly may have been, however, the truly revolutionary potential of cloning, from the perspective of medical science, wasn’t recognized until 1998, a year after Dolly’s birth was announced. That was when Thomson, by then a researcher at the University of Wisconsin-Madison, figured out how to harvest stem cells from human embryos left over from fertility treatments. Thomson was able to isolate and extract “pluripotent stem cells,” which are found in embryos only during the earliest stages of development and are capable of morphing into any manner of the body’s 200-odd different types of cells, from neurons and muscle tissue to blood and bone. By feeding a culture of these embryonic stem cells a precisely tailored chemical cocktail, Thomson could induce a process known as differentiation, triggering the embryonic cells to evolve into whatever type of cell he desired, be it liver cells for patients with liver disease or insulin-producing pancreatic cells for diabetics.
Pope John Paul II and other opponents denounced Thomson’s procedure as immoral because it involved the destruction of a human embryo—once the stem cells are harvested, the embryo is discarded. But scientists immediately recognized that Thomson’s discovery, married with Wilmut’s embryo-cloning technique, could revolutionize modern medicine.
The problem that such a coupling could solve was critical. Stem cell transplants had, in fact, been performed in leukemia patients for decades. The bone marrow stem cells used in such transplants are called “adult” stem cells. However, such stem cells, which are found in the parts of our body that constantly need regeneration (like bone marrow, skin and blood), have limited usefulness in medicine, because unlike the pluripotent stem cells that Thomson had harvested, adult stem cells have the potential to become only one thing. In other words, adult blood stem cells can only produce other blood stem cells.
Adult bone marrow stem cells from a healthy donor, when transplanted into a leukemia patient, will produce healthy bone marrow, potentially saving that patient’s life. But finding a donor with compatible DNA can be difficult, and even if a good donor is located, a patient’s immune system might reject the transplanted tissue, mistaking the bone marrow stem cells for foreign invaders.
The ideal solution? Replace a patient’s diseased cells with his own healthy cells—or ones that are an exact genetic match, which would be possible by using adult stem cells only if the patient had an identical twin. Or a clone. Or, perhaps more realistically, a supply of cloned pluripotent stem cells.
Using the process Ian Wilmut had developed to create Dolly, scientists theorized they could solve the tissue rejection problem once and for all (and eliminate the need to procure donated organs, blood and tissues) by creating just such a supply. They could do so by taking a skin cell from a sick patient and creating a cloned embryo reprogrammed with the patient’s healthy DNA. But instead of implanting the embryo into a womb to create an entire organism as Wilmut had done with Dolly, they could use Jamie Thomson’s stem cell extraction method to create an unlimited supply of pluripotent stem cells. Because these cells would be genetically identical to the patient’s, the challenge of finding donor cells would be overcome.
Thus, in 1998, the therapeutic cloning race began. But the problem with actually pulling this off was that scientists only knew how to clone a sheep. And the egg of a sheep is an entirely different microscopic beast than the egg of a human. This meant that those labs that opted to try to clone human embryos needed what would likely amount to thousands of human eggs with which to work. Considering the invasive, potentially dangerous procedure a woman must undergo to donate her eggs, those were going to be hard to come by. Indeed, as scientists sought to be the first to clone human embryos in the years that followed, the scarcity of eggs thwarted progress.
But there was another option. Someone could determine how to clone the embryos of the rhesus macaque monkey, which share 93 percent of their DNA with humans. With monkeys at the nation’s nine federally funded National Primate Research Centers, scientists could conceivably have access to more eggs, as opposed to relying on humans to donate theirs. But due to the expense (it would cost at least $10,000 a year to work with one monkey, but in each year 100 monkeys would be needed) and expertise that such research required, few scientists were interested. The only NPRC that opted to enter the race was Oregon’s, where a bright young researcher from Kazakhstan had just arrived.
Not long after Thomson’s discovery in 1998, Don Wolf had hired 37-year-old Shoukhrat Mitalipov, an unknown scientist who had just spent three years researching stem cells at Utah State University after earning his PhD in developmental genetics and stem cell biology from the Russian Academy of Medical Sciences in Moscow. Wolf had recruited the Kazakhstani scientist because he reminded Wolf of another brilliant postdoc he’d once overseen: Jamie Thomson.
Given the run of the lab, Mitalipov threw himself at the prodigious problem of combining Wilmut’s cloning technique with Thomson’s method, but using the embryos of primates instead. It wasn’t just the technical challenge that motivated him. One of Mitalipov’s friends in Russia had a son with leukemia. Later, his own mother would be diagnosed with Parkinson’s. Both diseases—theoretically—could be cured as a result of stem cell research.
“It became my obsession,” Mitalipov explains. “I knew I might not be able to save my mother, but my hope was that I’d one day help other people with this disease.”
Within the first few years of his research, Mitalipov successfully used Thomson’s method to extract pluripotent stem cells from natural monkey embryos flushed from the uterus; then he figured out how to trigger those stem cells to grow into colonies known as cell lines. Somewhat akin to sourdough starter, these stem cell lines, provided with the right nutrients, can be kept alive indefinitely and used to produce ever more stem cells, which in turn can be coaxed into heart tissue or skin tissue or neurons as needed.
However, for reasons that were mysterious to him, Mitalipov couldn’t successfully clone an embryo from which to create those stem cell lines. Using an ATM-sized machine called a micromanipulator, he could suck the DNA out of a monkey egg; and employing Wilmut’s method, he could insert the DNA from another monkey’s skin cell into that egg. But he couldn’t get the egg to reprogram successfully and become an embryo.
Scientists at private labs who also had begun working with both monkey and human eggs were caught in the same snag. In a study that was published in the journal Science in April 2003, Gerald Schatten, a University of Pittsburgh developmental biologist who had worked at the ONPRC in the late 1990s, confirmed what many in the field had feared. After trying hundreds of times to clone monkey embryos, Schatten concluded that it was impossible.
“It is almost as if someone drew a sharp line between Old World primates—including people—and other animals, saying, ‘I’ll let you clone cattle, mice, sheep, even rabbits and cats, but monkeys and humans require something more,’” Schatten told Science.
But in his lab in Hillsboro, Mitalipov plodded on, and in 2006, he made a breakthrough.
“The primate egg is one of the most fragile and sensitive cells in nature, and here we were, putting it through hell,” says Mitalipov. “Something needed to change.” He began to suspect that the “something” had to do with the dye he’d been using. To guide the needle of his micromanipulator during the reprogramming process, Mitalipov had to stain the egg’s nucleus with a dye that glowed under ultraviolet light. The dye and light hadn’t harmed the eggs of any of the 15 other animal species that had been cloned thus far, but given the fragility of the primate egg, Mitalipov began to wonder if he had found the process’s Achilles’ heel. But without the dye, how would he be able to see the nucleus or extract the DNA?
Mitalipov had an idea. In 2006, he borrowed an Oosight Imaging System, a computer that uses polarized light to take digital pictures of microscopic structures inside eggs. But instead of using it to take pictures, Mitalipov used it as a video camera to help him remove genetic material. Eventually it worked: The egg survived the reprogramming process and matured into an embryo that reached the blastocyst stage, meaning it had produced stem cells. But it would take Mitalipov over 300 more tries, using as many eggs from 14 different monkeys, to refine the process. Finally, in January 2007, he was able to transform the stem cells from a successfully cloned embryo into a cell colony and get it to develop into heart tissue that matched the skin cell donor. Two weeks later, the heart tissue began to beat.
“I thought, This is a historic moment,” he recalls. “Finally, I knew we had solved all these problems.”
Eager to share his discovery, he rushed home and told his wife and children. The kids demanded to see the heart their daddy had made from the monkey’s ear. He obliged. Back in the lab, he lifted up his 8-year-old daughter, Nargiz, and then his 5-year-old son, Paul, to see the pulsing cells.
You can hear their excitement in the video clip he shows during presentations.
“What is it, Daddy?” they both asked.
“This is magic,” he told his children. “This means we can cure lots of diseases and help millions of people, including Grandma.”
Mike Thomson and Wilmut before him, Mitalipov made the front page of the New York Times after he publicly announced his discovery in the journal Nature on November 15, 2007. In the Boston Globe, Reverend Thomas Berg, director of the Westchester Institute for Ethics and the Human Person, a Roman Catholic political think tank, warned that Mitalipov had eliminated the last barrier to cloning humans, an inevitability he deemed “one of humanity’s darkest endeavors.”
But just four days after Mitalipov’s discovery was announced, Jamie Thomson unveiled a discovery of his own. In the past several years—faced with the technical challenges of embryonic cloning, funding barriers and political opposition—the University of Wisconsin’s stem cell pioneer had begun exploring an alternative to embryonic cloning. By inserting a genetic engineering tool known as a retrovirus into an adult human skin cell, he was able to reprogram its DNA and coax it into behaving like an embryonic stem cell. He called these cells “induced pluripotent human stem cells” and proclaimed that his method had circumvented the need for cloning and the destruction of human embryos altogether.
Two weeks after the journal Science published Thomson’s results, the Weekly Standard ran the headline, “The End of the Stem Cell Wars: A Victory for Science, for the Pro-Life Movement, and for President Bush.” President Bush, in his 2008 State of the Union address, hailed Thomson’s breakthrough as the long-awaited answer to the divisive cloning debate, promising to fund the procedure exclusively and calling for a ban on “unethical” attempts to clone human embryos.
In the swell of publicity that followed, the normally reticent Thomson, who has since retreated from the public eye and who declined an interview with Portland Monthly, crowed to the Washington Post, “What a great bookend. Ten years of turmoil and now this nice ending. I can relax now.”
“It’s the most unbelievable medical advance in the last 25 years,” says Maureen Condic, a senior fellow at the Roman Catholic Westchester Institute who’s also an associate professor of neurobiology and anatomy at the University of Utah. “One little prick, and you get millions of skin cells that can be turned into stem cells. The only reason to clone now is that you’re likely to end up in an editorial in the New York Times.”
In an even more devastating blow to Mitalipov, Ian Wilmut, the “father” of cloning, declared that he, too, was abandoning the cloning process and adopting Thomson’s method, which he called “100 times more interesting” and “easier to accept, socially.”
Now, the University of Wisconsin is building a multimillion-dollar research center for Thomson. But despite Thomson’s popularity, there are at least a few scientists in the United States besides Mitalipov who still consider cloning a viable option. Robert Lanza, chief scientific officer at Advanced Cell Technology, a Los Angeles-based biotech firm that continues to pursue human embryonic stem cell cloning, warns that it’s too soon to write off Mitalipov’s research completely.
“Right now, we need to move ahead on both fronts and let the science play itself out,” says Lanza, who is also studying Thomson’s technique. “Despite the religious right wanting everybody to think that everything is solved, it’s not. We now have two possible ways to resolve this profoundly important problem, and it’s up in the air as to which is really going to succeed in doing this first.”
The primary problem with Thomson’s technique, says Lanza, is that it relies on retroviruses that the FDA would never allow to be used in humans. Mitalipov worries that if Thomson’s stem cells were turned into bone marrow and transplanted into a patient, it would take only one rogue stem cell to revert back to its pluripotent state and begin to spread through the patient’s body, becoming cancer.
“Because of the ethical debate,” says Mitalipov, “science is playing politics. If you use these in patients, it would be pure homicide.”
But, says Lanza, assuming Mitalipov’s protocol could be reproduced in humans, the technique’s fundamental hurdle is that it relies on human eggs. Lanza points out that more than a year ago his company ran over 100 ads asking for egg donors, but only one candidate actually went through the procedure.
Mitalipov notes that in the year since he announced his breakthrough, he’s reduced the number of eggs required to create a blastocyst from 150 to 15; and he believes he can reduce this number to 2 or 3 eggs. If he could make cloning that efficient, he could rely on the supply of leftover eggs from in vitro fertilization procedures—because such eggs are typically discarded anyway.
But what Mitalipov would really like to do is discover the as-yet-unknown protein inside the human egg that gives rise to stem cells. With that knowledge, he could do away with the egg entirely and use the protein alone to manufacture stem cells, essentially merging his advance with Thomson’s—but using the egg’s natural protein to do the job instead of mucking around with cells artificially created by genetic engineering.
“Nature has developed this system over millions of years,” says Mitalipov. “Instead of reinventing the wheel, we need to understand how it’s done in the egg.”
To do that, he’d like to start cloning human embryos, but federal law prohibits the National Institutes of Health (NIH) from funding research that involves human cloning. Unless the President enacts an outright ban on human cloning, Mitalipov can always set up a lab with private funding. But no private donors have knocked on his door.
And it looks like OHSU has no plans to fund Mitalipov either. Nor will he be receiving an invitation to set up a private lab from Markus Grompe, director of the Oregon Stem Cell Center, an OHSU-based stem cell research lab that was established in 2004.
“I don’t control Shoukhrat and I don’t control the university, but they know my view on this,” says Grompe, who is also a fellow at the Roman Catholic Westchester Institute. “This could be a real negative for OHSU, because the pro-life community is going to view [it] as a problem institution. They may be a minority, but they’re a vocal minority. Do you really want to take that chance?”
Facing a wall of opposition in the United States, Mitalipov recently decided to go overseas and give up his hard-won secrets.
In January and February, Mitalipov went to England to train researchers at Newcastle University’s Centre for Life, a stem cell research lab that’s preparing a first attempt at cloning a human embryo. In exchange for Mitalipov’s help, Newcastle agreed to share future patent royalties with OHSU.
“I had no choice,” says Mitalipov.
Meanwhile, hoping to steer Mitalipov’s cloning research in a more ethically palatable direction, Grompe’s pushing a new process called “altered nuclear transfer.” The technique is essentially the same as Wilmut’s cloning method, but before the cloning begins, the proteins from the donor cell are altered so that the resulting “biological entity” would yield stem cells but would never mature into a viable embryo. Theologians have given the technique their blessing, allowing that since such embryos could never be implanted, they wouldn’t count as potential human lives.
“You have to play the games,” Mitalipov sighs. He then backpedals, insisting that Grompe’s preferred method will provide him with yet more insights into the mystery of the egg. “I wouldn’t do [altered nuclear transfer] just to please those who were critical about the ethics of cloning. To me, what’s important is the science behind it. That’s our master.”
But in the case of human cloning and stem cell research, it’s tempting to think that the master has been relegated to a slave.




