This Medford Clinic Is Developing a Coronavirus Vaccine, and You Wouldn’t Even Know It
There’s nothing extraordinary about the structure that houses the Clinical Research Institute of Southern Oregon. The site, a beige building with an arched entryway and glass doors, sits just east of Crater Lake Highway’s wisps of everyday traffic. And yet, the fate of the world might just lie here, on Hollyhock Drive in Medford.
Since July, the institute has been one of the staging grounds for a quest to find a vaccine for the novel coronavirus, which causes a disease that has killed more than 700 people in Oregon, more than 230,000 in the US, and 1.2 million worldwide. And inside, Gregg Lucksinger, the interim medical director of the institute, taps a business card against an oval wooden table in a brightly lit corner room. He leans back in an office chair and reminisces about the lessons learned in Liberia, where he worked in an Ebola treatment unit in 2015. He hopes those lessons will aide him and his colleagues now.
“In [Liberia], the epidemic overwhelmed health systems.... It was really fascinating to see that,” he says. “And that’s what we’re struggling with in the United States to prevent from happening. We’ve been really on the edge in a few places.”
Lucksinger’s work in clinical research began in Austin, Texas, during the HIV epidemic of the 1980s. He went on to help develop vaccines and/or therapeutics for HIV, hepatitis C, and Ebola.
Now, another complicated and highly contagious virus threatens humanity. Lucksinger and the institute in Oregon are working on two Phase 3 COVID-19 vaccine trials. The first, produced by biotech startup Moderna (which has been proven to reduce the risk of COVID-19 infection by 94.5 percent), has wrapped up its enrollment period and moved to data collection, a process by which research coordinators follow up with volunteers to see if they’ve developed symptoms of or contract the coronavirus as they go about their daily lives. This stage could take a year or two. The second trial is for a vaccine produced by Novavax. Under the White House’s Operation Warp Speed (OWS), a $10 billion initiative, COVID-19 vaccine and therapeutic trials have been accelerated, compressing what typically takes years into months. By running various processes in parallel and increasing the number of volunteers from a couple hundred to at least 30,000 for each trial, OWS hopes to have a vaccine by early 2021.
OWS’s accelerated timeline means the car is being assembled on the highway.
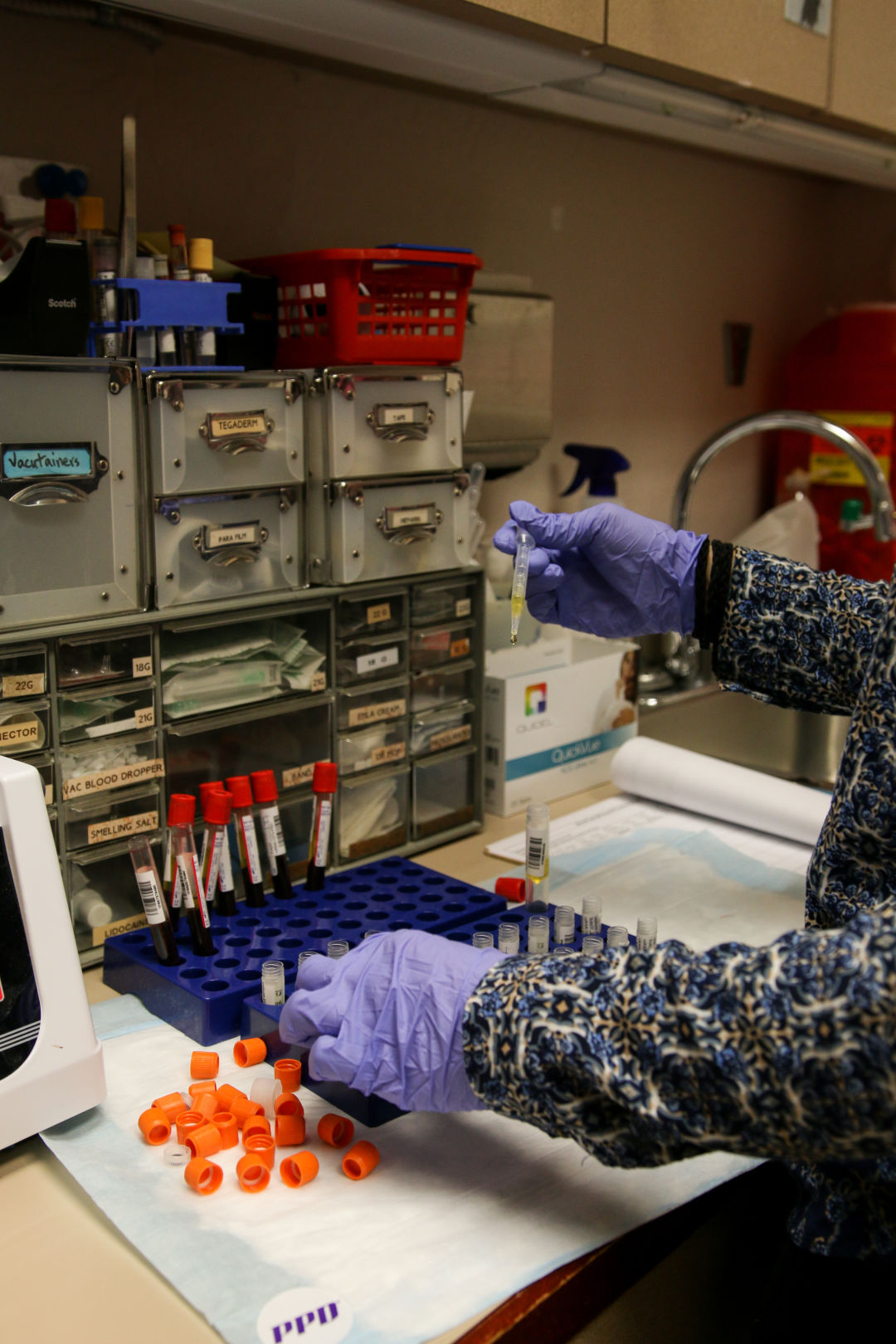
Since July, the Clinical Research Institute of Southern Oregon in Medford has been one of the staging grounds for a quest to find a vaccine for the coronavirus.
Image: Gabriel Granillo
Lucksinger and his crew—as well as about a dozen other Phase 3 coronavirus vaccine trial sites, and more than 100 research trials actively working on vaccines and treatments, across the US—are currently assessing the efficacy of the vaccines by injecting paid volunteers with either the vaccine or a placebo. The data from that research will be reviewed, and hopefully approved, by the FDA. And while all of that is happening, medical companies are already in production mode. “In pharmaceutical and vaccine development there is a lot of risk. I mean, most projects don’t make it,” Lucksinger says, “but this is so urgent, because of the number of people that are dying, the number of people that are getting sick, and the impact on society economically, psychologically is just so profound, that they started mass producing some of these vaccines before we were even in Phase 2.”
But if the vaccine proves ineffective, down the drain it goes, says Dr. Paul Evans, CEO of Velocity Clinical Research, based in North Carolina. He says the sooner the Medford institute can produce the data the FDA needs, the sooner proven effective vaccines can start saving lives.
Evans has played a major role in organizing and conducting the trials currently happening in Medford. For 30 years, he has been involved in clinical research, but this research on the coronavirus, he says, “is probably the most important work we’ll ever do in our careers.”
“The clock’s ticking,” Evans says. “It’s not overstating it to say this is critical for mankind. I don’t think we’ve ever done a trial where we’ve been able to say that, but we can in the case of this one.”

The Clinical Research Institute of Southern Oregon
Image: Gabriel Granillo
Medford is a gateway town, divided by I-5 and US 99, which lead to the award-winning vineyards that decorate the hillsides up north, and to the California forests down south. A town, today, contending with COVID and historic wildfires that have destroyed entire communities in Southern Oregon who now seek shelter in fairgrounds and RV parks and hotels.
Since start of the pandemic, Jackson County, of which Medford is the county seat and most populous city, has fared relatively well compared to more urban counties. Multnomah County, for example, has just under four times the population of Jackson but has reported more than 20 times as many coronavirus deaths.
The Almeda and Obenchain Fires, which burned most of Talent, Oregon—a town of 6,000 people seven miles south of Medford—created a rising concern that new cases may begin appearing, with evacuees seeking shelter with family and friends, or in motels.
While they changed the dynamics of Jackson County’s COVID-19 response, the fires had little effect on the vaccine trial in Medford. “Things [at the institute] continued pretty much like clockwork,” says Lucksinger. “These challenges are common in studies.”
Another challenge experienced by most clinical trial sites, Lucksinger says, is getting a representative slice of the population. “In almost all clinical trials that are done in the United States, people of color are usually vastly underrepresented, and sometimes that has big consequences”—namely, that a vaccine or “tool” might be effective for only one portion of the population. The goal, Lucksinger says, is to make a tool accessible for everyone.
“It’s great to take care of sick people and get them well, but I like to develop tools that then thousands of people can use to help sick people,” says Lucksinger. “Even if I’m asleep or on vacation, or even some day when I’m dead, just knowing that those tools that I helped developed will continue to help people all the way around the world, to me, is just super gratifying.”
But until that tool is developed, which may not happen until next year, more people will die from the coronavirus. Medford and Southern Oregon will clear the ash and debris left over from an unprecedented wildfire season that burned more than a million acres of land in the state and displaced thousands of Oregonians.
And Lucksinger and his team of research coordinators will quietly plug away in a beige building on Hollyhock Drive.
Correction: A previous version of this article stated Gregg Lucksinger had worked in an Ebola treatment unit in 2013. Lucksinger worked in the unit in 2015.




